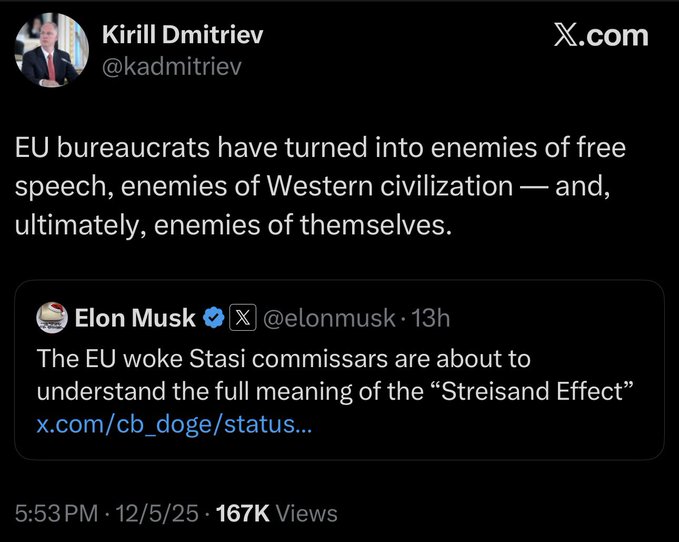
MS-DOS без Микрософта
Dec. 14th, 2025 12:10 amРазочаровался я в SvarDOS и DR-DOS. Работаешь так, работаешь, проверяешь иногда файловую систему, вдруг чего подпортилось, и неожиданно:

И чего дальше с этим делать? Переформатировать диск заново, до следующей оказии? Нет, такой операционкой пользоваться не годится. Надо искать альтернативу.
Основной способ обмена файлами между писишкой и внешним миром у меня - перетыкание SD карточки (которая диск C: и прочие) в линуксный лаптоп. Линукс поддерживает длинные имена в файловой системе FAT. При копировании файлов я стараюсь, чтобы имена были досовские, то есть 8.3 uppercase. Но мало ли где могу промахнуться. Одно неловкое движение - и CHKDSK сломается навсегда. Нужна версия MS-DOS с надёжной поддержкой длинных имён.
Оказывается, такая существует, и называется она... Windows 98. 😀 Нижний "досовский" уровень тех окошек представляет собой фактический старый добрый MS-DOS 7.1, но улучшенный длинными именами файлов. Проблема только в том, что Микрософт никогда не выпускал тот DOS в виде отдельного продукта.
Оказывается, нашлись умельцы, которые разобрались в этом деле и выпустили неофициальный релиз MS-DOS 7.10. Скачать можно отсюда: winworldpc.com/download/40c395e2-8093-c2a9-18c3-9a11c3a4efbf
Ставится это дело с двух флопиков 3.5". Вот пример загрузки после установки на диск XTIDE:

( +3 )
Буду теперь использовать этот ДОС как основной вместо родного микрософтовского на 486 машинке. Жаль только, на XT-шке он не грузится. Затыкается сразу где-то в бутсекторе.

И чего дальше с этим делать? Переформатировать диск заново, до следующей оказии? Нет, такой операционкой пользоваться не годится. Надо искать альтернативу.
Основной способ обмена файлами между писишкой и внешним миром у меня - перетыкание SD карточки (которая диск C: и прочие) в линуксный лаптоп. Линукс поддерживает длинные имена в файловой системе FAT. При копировании файлов я стараюсь, чтобы имена были досовские, то есть 8.3 uppercase. Но мало ли где могу промахнуться. Одно неловкое движение - и CHKDSK сломается навсегда. Нужна версия MS-DOS с надёжной поддержкой длинных имён.
Оказывается, такая существует, и называется она... Windows 98. 😀 Нижний "досовский" уровень тех окошек представляет собой фактический старый добрый MS-DOS 7.1, но улучшенный длинными именами файлов. Проблема только в том, что Микрософт никогда не выпускал тот DOS в виде отдельного продукта.
Оказывается, нашлись умельцы, которые разобрались в этом деле и выпустили неофициальный релиз MS-DOS 7.10. Скачать можно отсюда: winworldpc.com/download/40c395e2-8093-c2a9-18c3-9a11c3a4efbf
Ставится это дело с двух флопиков 3.5". Вот пример загрузки после установки на диск XTIDE:

( +3 )
Буду теперь использовать этот ДОС как основной вместо родного микрософтовского на 486 машинке. Жаль только, на XT-шке он не грузится. Затыкается сразу где-то в бутсекторе.